Alkane Nomenclature:Learning how to name alkanes will
be crucial in your understanding of organic chemistry. All organic compounds are named
following a certain set of rules set out by the IUPAC. By memorizing these rules, you
should be able to name all compounds.
To name alkanes....
(follow these set of rules)
1.) Count the longest carbon chain
If the carbon chain is:
- 1 carbon: named "methane"
- 2 carbons: named "ethane"
- 3 carbons: named "propane"
- 4 carbons: named "butane"
- 5 carbons: named "pentane"
- 6 carbons: named "hexane"
- 7 carbons: named "heptane"
- 8 carbons: named "octane"
- 9 carbons: named "nonane"
- 10 carbons: named "decane"
Note that organic chemists, being lazy, will often not label hydrogens or carbons. For
instance,
 would be
hexane (each of the "points" is assumed to be a carbon)
would be
hexane (each of the "points" is assumed to be a carbon)
2.) Number the carbon chain, starting with the end closest to the substituent.
Always try to get the lowest number possible.
If there are two substituents, name the carbon chain so that it has the lowest possible
number.For instance, check out this molecule:
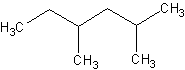
Would this compound be numbered from the left or right? The answer is that it would be
answered from the right. If the molecule was named from the left,
you would have substituents on carbons 3 and 5. Numbering from the right, the subsituents
are on carbons 2 and 4.
Once you have determined which direction you will name, you will have to write down the
molecule.
3.) Name and number the substituent
When naming, name the substituent before the parent chain. Name this compound:

You would name this 2-"substituent" hexane. You would not name it
5-"substituent" hexane because 2 < 5.
These are the common substituents in organic chemistry:
- (CH3--): methyl
- (CH3CH2--): ethyl
- (CH3CH3CH2--): butyl
- Notice the pattern yet? You take the full name, for instance, hexane, drop the
"ane" and add "yl," so a carbon chain with (CH3CH3CH3CH3CH3CH2--)
would be called "hexyl"
Now, when you have two of the same substituents on a molecule, use these common
prefixes:
- two of the same substituent: di
- three of the same substituent: tri
- And so it keeps goin....
Name this compound:
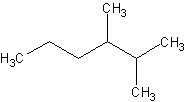
- The longest carbon chain is 6 carbons. Hence the parent chain is named
"hexane"
- Two subsituents are located on carbons 2 and 3, not 4 and 5.
- There are two "methyl" substituents
- Hence, we have the name, 2,3-dimethylhexane
|
If you have two substituents that are not the same, then you must name
them both before the parent chain, listed alphabetically. Note that the prefixes used to
designate number (i.e. di and tri) do not affect the alphabetization.
Name this compound:
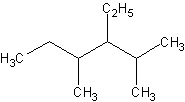
- The longest parents chain is 6 carbons, hence its parent name is "hexane"
- I spy, with my own eyes, three substituents! There are two methyl and one ethyl group.
How should I number them? Well, seeing as to how I can get 2,3,4 instead of 3,4,5, I shall
name them from the left.
- So the substituents are 2,4-dimethyl and 3-ethyl. Note that "e" in ethyl
precedes "m" in "methyl. The "di" doesn't affect the
alphabetization.
- Finally, we name the compound as
- 3-ethyl-2,4-dimethylhexane.
|
You're done!!
HAHA, JUST KIDDING!