Atoms:
By TakalahTopics Covered
This section introduces some basic concepts of chemistry.
Law of Multiple Proportions
The Law of Multiple Proportions simply states: When two elements form a series of
compounds, the ratios of the masses of the second element that combine with 1 gram of the
first element can always be reduced to small whole numbers.
For those of you who are lost, it just means that only multiples of an amount of one
element can react with a constant amount of another in a two element compound. For
example, here is a table comparing three compounds of nitrogen and oxygen.
| Compound |
Amount of Nitrogen that reacts with 1
gram of oxygen |
| Compound A |
1.750 g |
| Compound B |
.8750 g |
| Compound C |
.4375 g |
See the smallest number, .4375? It divides into the other numbers with
no remainder. That is, B/C is 2, and A/C is 4. This just shows that molecules are made up
of finite sized units, or atoms. You can't have 2.3 atoms combine with 1 atom. Same thing
here.
There is no way to know from this data alone exactly the ratios of the molecules. You
could take the base unit to be any one of the numbers. Let's do two examples.
Using .4375 and ratio 1N : 1O (Compound C):
Let's call .4375 the ratios of the molecules 1N : 1O. Since Compound B contains twice
as much N, you could say that the ratio there is 2N : 1O. Since Compound A contains four
times as much N as Compound C, you could say that the ratio is 4N : 1O.
Using .8750 and ratio 2N : 2O (Compound B):
Now you say that .8750 is your base number, and the ratio 2N : 2O. What will the ratio
of Compound C be? You know that there is half as much Compound C as in Compound B, so just
reduce the N by one-half; 1N : 2O. And since you know there is 4 times as much in Compound
A, it's 4N : 2O. It's that easy.
As you can guess, there is an infinite number of situations that satisfy the table.
Dalton's Atomic Theory
These are pretty straightforward and require no explanation. Although you don't use
these on tests or anything, it's good stuff to know (not to mention our whole
understanding of chemistry is based on this):
- Each element is made up of tiny particles called atoms.
- The atoms of a given element are identical; the atoms of different elements are
different in some fundamental way or ways.
- Chemical compounds are formed when atoms combine with each other. A given compound
always has the same relative numbers and types of atoms.
- Chemical reactions involve reorganization of the atoms--changes in the way they are
bound together. The atoms themselves are not changed in a chemical reaction.
These ideas form the basis of what a lot of AP Chem is all about; chemical reactions.
It's nothing more than breaking some bonds and making some bonds, to put the atoms in a
different order to create new things.
Intro to Atomic Structure
An atom consists of a nucleus and electrons orbiting around it. The nucleus is made up
of positively charged particles called protons, and neutral particles called neutrons. The
electrons are negatively charged particles.
Here is a simplified picture:
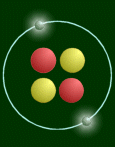
This atom has two protons (red), so it is a helium atom.
One thing that pictures like the above cannot express is the relative sizes of the
nucleus and the atom. If the atom was really that big (diameter 108 pixels), then the
nucleus would be 1/100th of a pixel big! Or on a bigger scale, if the nucleus was the size
of a pea, then the atom would be as wide as a football field. Another thing is mass.
Virtually all the mass is concentrated in the nucleus. The 'pea' would weigh 250 million
tons!
There are two numbers to know about any atom. One is the atomic number, which is the
number of protons (and electrons in a neutral atom), and the atomic mass number, which is
how much the thing weighs. One proton's weight is close to one neutron's weight which is
close to one. Electrons weigh virtually nothing when compared to the huge protons, so they
don't affect the total mass. So the atomic mass number is simply the number of protons
plus the number of electrons.
The notation of writing atoms is like this:
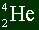
The top number represents the mass number.
The bottom number represents the atomic number.
Some things to remember while solving these types of problems:
(Note: Problems of this simplicity will not be on the AP test, I guarantee that. We are
not so lucky. But you will be tested in class on this stuff.)
- The bottom number is always the number of protons.
- The bottom number is also usually the number of electrons, but only in a neutral
element. Ions come later.
- The number of neutrons is always the top number minus the bottom number. All
you're doing is taking the total mass, taking away the portion that is due to the protons,
and what you got left is the neutrons. Likewise, if you know number of neutrons and
protons, to find this number all you got to do is add.
Example:
| Sample Problem 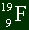
Here is a sample atom. How many electrons, protons, and neutrons does it got?
Solution
The bottom number is always the number of protons you have. So there are 9 protons.
It's a neutral atom, by the fact that there are no plus or minus signs anywhere, so you
can assume there are 9 electrons as well. There are 19 total nucleons (protons and
neutrons) in the nucleus, and 9 are protons. So there are 10 neutrons. Easy. |
Molecules and Ions
Compounds are made up of two or more atoms held together in some way. Although I'll go
way more in depth later, atoms can be bonded together by both sharing electrons or
one taking electrons away from the other.
Those compounds that share form a covalent bond, and the resulting formation is
a molecule. An example is water, H--O--H. Each H shares its electron with the O, so
that the O has two shared electrons, completing its valence shell. Another is methane, CH4,
which has each H sharing an electron with C, and C sharing a total 4 electrons with the
H's.
A molecule acts as a unit by itself. It moves together. To represent a
molecule, you can use either a ball-and-stick model or a space filling model. Examples of
H2O are shown below.
 |
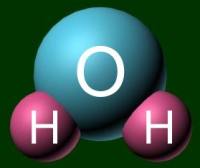 |
Ball-and-stick model |
Space filling model |
As a general rule, a covalent bond results when two non-metals are put
together.
Let's consider the case of NaCl. Chlorine has a higher affinity for electrons than
sodium, and so it takes one away, shown by the following:
Cl + e- -> Cl-
This shows that the electron joins up with the chlorine, and becomes a negatively
charged ion--also known as a anion. But where did this electron come from? From the
sodium, of course:
Na -> Na+ + e-
This shows that the normal sodium atom gives off an electron--and becomes a positively
charged ion in the process--also known as a cation.
Since these charges have opposite signs, they attract. The positive Na+ and
the negative Cl- form a ionic solid, also a salt. (Keep in mind that a
salt refers to any ionic solid, not just our example of table salt. Ions don't have
to be just one atom; they can be polyatomic, or consisting of many atoms. Some
examples are the anion C2H3O2- and the cation
NH4+. A general rule, not to be followed strictly; if a compound has
a metal and non metal in it, it's ionic. One of many exceptions is NH4Cl; I
guess the best rule to follow is to look for those ions!
One of the most annoying things in chemistry is memorizing ions. Once every week, my
class would have to memorize ten ions and have an Ion Quiz. Although you might forget
them, it is a good idea to review them while preparing for the test. I have included an
ion table in the Tables section.
Compound Naming
I hated this part in Chem I. But it's not too bad. On a test, a question might be to
mix dinitrogen pentoxide with something else. No problem, but what's that made of? This
section will show you how to take a chemical structure and give a name to it, and vice
versa.
Binary Ionic Compounds
These are just compounds made up of two different ions, like sodium chloride. All you
do is: Put the name of the cation, followed by the name of the anion. That's it.
Example:
| Compound |
Name |
Ions |
| KI |
Potassium Iodide |
K+, I- |
| CaS |
Calcium Sulfide |
Ca2+, S2- |
| MgO |
Magnesium Oxide |
Mg2+, O2- |
| Li3N |
Lithium Nitride |
Li+, N3- |
For single one-atom ion compounds such as above, you keep the name of
the cation, and change the ending of the anion to -ide. Other examples are hydrogen =>
hydride, carbon => carbide, Fluorine => Fluoride, you get the idea.
One more thing to consider is the charge on the metal atom. If it can have only one
possible value (such as +1 for sodium and +2 for magnesium above), then the normal name is
fine. If the metal can form cations with different charges, like iron and tin, then you must
put the Roman Numeral of it in parenthesis right after the name of the cation. Here are
some examples:
| Metal Ion |
First half of compound name |
| Fe2+ |
Iron (II) |
| Fe3+ |
Iron (III) |
| Pb2+ |
Lead (II) |
| Pb4+ |
Lead (IV) |
How do you know if to use roman numerals or not? Generally, if the metal
is a transition metal (belonging to the three row skinny middle part of the periodic table
up to the metalloids), then you need to use it. Some exceptions are aluminum (+3), zinc
(+2), silver (+2), and cadmium (+2). Even though these are transition metals, they have
only one possible ion. These metals and the metals in the first two columns need not use
it, in fact, don't use them.
Another thing; mercury is really weird here. The two possible ions with mercury are Hg2+22+.
The difference when writing this is that Mercury (II) for the first, and Mercury (I).
Because there is a total of +2, and there are two.
Nothing to it. Before I get to the sample problems, there is one more important point
to all this: Compounds don't have charge. So when you add up all the charge from the ions,
they must equal zero. And if they don't, you must balance it. Note that in the first
table, the first three ions, when charge is added up, give zero. The last one is
different. It still must add up to zero, but note that N is -3, but Li is only +1. It's
common sense that you need three Li atoms to cancel out the -3 in N, hence the formula.
Example:
| Give the name of these compounds: a.) CuCl b.) NaF c.) MnO2
d.) FeBr2 e.) Fe2O3
Answers
- By the rules, this is Copper Chloride. But copper can have different charges, so you
must figure what the charge on this one is. Let's see: you know chloride can have only one
value, and that's a -1. So if they must add up to zero, Cu must be +1. The answer is
copper (I) chloride.
- This is sodium and fluorine, so the name is sodium fluoride. Sodium can only be +1, so
there is no need for roman numerals.
- The name is Manganese Oxide, but a roman numeral is necessary, since it's a transition
metal. In this case, manganese is a transition metal, so it needs a roman numeral. Let's
start with oxygen: Each oxide ion has a charge of -2, and there are 2 of them. So the
total charge due to oxide is -4. The single manganese ion must compensate for this, so its
charge must be +4. The name is manganese (IV) oxide.
- Same here. It needs a roman numeral. Br is -1, and 2 of them, so Fe must be +2. Name is
Iron (II) Bromide.
- e.This one's a little bit different, but the same concepts apply. You start with the
oxygens. 3 of -2 ions make it a total of -6 charge due to oxide. So the two irons must
compensate. If two of them must equal +6, each must be...+3!!! It should be no surprise.
So the name of this is Iron (III) Oxide.
|
Polyatomic Ions
Many compounds don't have just one element for an ion. You can have multiple atoms of
different types in an ion. One of the most common ones are oxyanions, an anion containing
one atom and one or more oxygen atoms. They usually have a series associated with them,
with the number of oxygen atoms varying. The naming system is simple; the thing you must
memorize for each element is where the naming system begins.
Many compounds don't have just one element for an ion. You can have multiple atoms of
different types in an ion. One of the most common ones are oxyanions, an anion containing
one atom and one or more oxygen atoms. They usually have a series associated with them,
with the number of oxygen atoms changing. The naming system is simple; the thing you must
memorize for each element is where the naming system begins.
| Ion |
Name of Ion |
| ClO- |
Hypochlorite |
| ClO2- |
Chlorite |
| ClO3- |
Chlorate |
| ClO4- |
Perchlorate |
Very easy stuff. Note that the charge is the same for all; it's not a
coincidence. All members of a oxyanion series have the same charge. Other series are the
phosphates and the sulfates. The trick is remembering for each series where it starts.
Once you remember that, then it's easy to get the other names if you know the naming
rules. Like for the chlorines, remembering that 3 oxygens is the chlorate form is all that
you need. Then you know that if you add one oxy, it becomes perchlorate, take away one,
then its chlorite, and so on.
The hardest part for some people, if any, when solving polyatomics is that an ion is a
unit by itself. Like for example, when looking at Na2SO4, don't
think of it as Na+, S2-, and O2-. That is wrong
and will give you wrong answers. The real way to look at it is Na+ and SO42-.
You can't break up that ion! And remember that when you need more than one polyatomic ion,
use ()'s! If two hydroxides are needed to balance that 2+ calcium, use (OH)2
not OH2!
All other rules hold. You need roman numerals for transition metals, and the total
charge must equal 0.
Covalent
The other binary compound is called covalent. If you have noticed, ionic compounds
generally are metals joined to non metals, with exceptions. Covalent compounds are two non
metals put together. This is by far the easiest way to name compounds. Just add the
appropriate prefix to each of their names, along with the -ide suffix on the second
element.
| Number of Atoms |
Prefix |
| 1 |
mono- |
| 2 |
di- |
| 3 |
tri- |
| 4 |
tetra- |
| 5 |
penta- |
| 6 |
hexa- |
| 7 |
hepta- |
| 8 |
octa- |
| 9 |
nona- |
| 10 |
deca- |
One other little minor rule; if there is only one of the first element,
don't use mono-. It's reserved only for the -ide part.
Examples:
CO2 => Carbon Dioxide
CO => Carbon Monoxide (note that it's not Monocarbon Monoxide, that sounds crazy! :)
N2O5 => Dinitrogen Pentoxide (change the a in 'penta' to o,
awkward to have 'a' and 'o' next to each other)
SF6 => Sulfur Hexafluoride
Don't get this mixed up with the ionic stuff. You might be tempted to write Fe2O3
as Di-iron Trioxide because it's very easy, but that is very wrong. This prefix system is
only for covalent compounds. Which is why students tend to like covalent compounds better
than ionic compounds.
Using all that info above, you should be able to do the reverse of what we've been
doing; that is, you should be able to supply a formula given a name. Let's try some:
Example:
Find the formula for each chemical below.
- Sodium sulfate
- Vanadium (V) fluoride
- Mercury (I) chloride
- Tin (II) fluoride
- Cobalt (III) nitrate
Answers
- The ions are Na+ and SO42-. But Na has only +1, and SO4
is -2. So you need two Na's to make up for that -2. The answer is Na2SO4.
- The ions are V5+ and F-. You know the +5 of vanadium by the (V).
So you need 5 fluorines to make up for that +5. The answer is VF5.
- Mercury is the weird ion. Since it's (I), then the ion must be Hg22+.
And since there is a +2, you need two Cl-'s to balance. The answer is Hg2Cl2.
- Tin is +2, so you need 2 F's to balance. Answer is SnF2.
- Cobalt is +3, and nitrate is -1. Need three nitrates to balance, so answer is Co(NO3)3.
|
That's it to this section. Go try the practice problems
now!
Copyright ©1999 by Takalah. All
rights reserved. This concepts portion was last updated on September 4, 1999