Atomic Structure and Periodicity
By Cassidy MullenELECTROMAGNETIC RADIATION
Electromagnetic radiation is one of the ways energy travels through space.
This includes X-rays, gamma rays, ultraviolet light (or UV), infrared light, microwaves
and radio waves. Visible light also makes up a small fraction of what is known as the electromagnetic
spectrum. The electromagnetic spectrum is the spectrum of all possible types of
electromagnetic radiation.


The Visible Spectrum
Although gamma rays may be vastly different from visible light, they both exhibit the
same type of wave behavior. All types of electromagnetic radiation travel at the speed of
light in a vacuum too.
The three main characteristics of a wave are wavelength, frequency, and speed. Wavelength
is the distance between two consecutive peaks or troughs. It is symbolized by the Greek
letter lambda, l . Frequency is the number of waves
(cycles) that pass a given point in one second. It is symbolized by the Greek letter nu, n . Speed is defined as cycles per second, or hertz,
abbreviated Hz.
Wavelength and frequency are inversely related. This is shown by the formula
c = (l)(n)
In which n is the frequency, l is
the wavelength, and c is the speed of light (2.9979 ´
108 m/s).
THE NATURE OF MATTER
Around 1900, everyone believed that energy and matter were two different things
altogether. Matter was thought to be made of particles (atoms) and energy was thought to
be made of light (electromagnetic radiation). Particles had mass while energy occurred in
waves and was massless.
That changed in 1900 when Max Plank, a German physicist, studied the emission of
radiation from solid objects. He observed that the physics of his day could not describe
the results he was obtaining. He postulated the theory that energy can be only gained or
lost in whole number multiples of the quantity hn ,
where h is Plank’s constant. Experiments determined the constant to be
equal to 6.626 ´ 10-34 J×
s. Therefore, the change in energy for a system can be shown by the equation
DE = nhn
where n is an integer (1, 2, 3, …).
With this, a new set of thinking came about. Before it was thought that a transfer of
energy was continuous. Now it was realized that energy could only occur in
"packets" called quantum.
Albert Einstein was next, proposing that even electromagnetic radiation was quantized.
He suggested that electromagnetic radiation could be viewed as a stream of particles
called photons. The energy of a photon could be described by the equation
Ephoton = hn = hc/l
where h is Plank’s constant, n is the frequency,
and h is the wavelength.
In his special theory of relativity, he showed that energy has mass. This
is shown in the formula
E = mc2
where c is the speed of light, m is the mass, and E is the energy.
The proof of energy having mass is shown if you rearrange the formula in the following
form:
m = E / c2
Since you know that
m = E / c2 = (hc/l)
/ c2 = h / lc
one can clearly see de Broglie’s equation,
l = h / mv
v = velocity, not frequency
This allows us to calculate the wavelength for a particle. It also proves that
particles do behave as waves.
In summary, it was now found that energy is quantized, meaning that it can occur only
in discrete units called quanta. It was also discovered that energy does contain mass.
This is known as the dual nature of light.
BOHR’S ATOMIC MODEL
In 1913, Niels Bohr, a Danish physicist, developed a quantum model for the
hydrogen atom. By combining classical physics and some theories of his own Bohr was able
introduce a revolutionary model for all atoms.
Previous atomic models had the negatively charged electrons orbiting the positively
charged nucleus. However, this is inaccurate because this would cause the electrons to
eventually "fly off" the atom. The man who invented this model of the atom,
Ernst Rutherford, knew that this was true, but couldn’t think of a better way to
describe an atom.
Using the spectrum of hydrogen, Bohr found that when a prism diffracted the light color
was displayed at only four discrete increments.
He saw that these results would fit his model if he assumed that the angular momentum
of the electron could only occur in certain increments. Angular momentum equals the
product of mass, velocity, and orbital radius. Using this he assigned the hydrogen atom
energy levels consistent with the hydrogen emission spectrum.
This gave way to Bohr historic equation for the energy levels available to the electron
in the hydrogen atom. The n is an integer. The larger the value of n, the
larger is the orbital radius. Z is the nuclear charge.
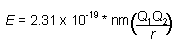
Bohr’s equation was able to accurately calculate the energy levels for the
hydrogen atom. Each energy level pertains to an electron in an exited state. It will move
up to higher energy levels, but when it goes back to its ground state (where n=1)
or to a lower level orbital it emits energy. That is what Bohr viewed when he saw the
spectrum of hydrogen.
To find the total change in energy once subtracts the energy in the initial state minus
the energy in the final state.
DE = Efinal - Einitial
If a negative sign appears as the result, that means that the atom lost energy and thus
is in a more stable state. However, if you insert this value into a separate equation, use
the absolute value of the change in energy.
The next equation’s purpose is derived from Bohr’s formula. It determines the
energy used for an electron moving from one level (ninitial) to another
level (nfinal).
-2.178 * 10-18 J ( 1/(nfinal)2) - (1/(ninitial)2))
However, it was soon found that Bohr’s model does not work for other atoms. Later
models, however, would explain atoms other than hydrogen.
QUANTUM MECHANICS
It was now known that Bohr’s model would work for hydrogen and hydrogen only.
A new model was needed. This became known as quantum mechanics. As de
Broglie’s equation showed that electrons acted as waves. Many physicists now tried to
attack the problem of atomic structure by using the wave properties of an electron. To
them the electron bound to the nucleus seemed similar to a standing wave.
Take a stringed instrument. The string vibrates to produce a musical tone. The waves
are "standing," because they are stationary; the waves do not travel along the
string. Now put this into the hydrogen atom, with an electron acting as a standing wave.
One physicist, Erwin Sgrödinger, used the formula
H y = E y
to illustrate the wave properties of an atom. The formula is in that form because the
math is too complicated to be detailed here. The y is called
the wave function. It is a function of the x, y, and z points
on a three-dimensional graph. The H represents a set of mathematical operations
called an operator. The E represents the total energy of the atom. When this
equation is analyzed in its entirety, each solution consists of a wave function (y ) that is characterized by a particular value of E. A
specific wave function is often referred to as an orbital.
To show this, let us concentrate on the hydrogen atom. The wave function corresponding
to the lowest energy is called the 1s orbital. Remember that an orbital IS NOT a Bohr
orbit. Th electron is the 1s orbital is not moving around the nucleus in a circular
motion.
Here is a quick point. According to Werner Heisenberg "there is a fundamental
limitation to just how precisely we can know both the position and the momentum of a
particle at a given time. This is known as the Heisenberg uncertainty principle. In
the formula
Dx * D(mv) >
h/4p
where Dx is the uncertainty in a particles position
and D(mv) is the uncertainty of a particle’s
momentum. This all means that one cannot simultaneously find a particle's position and
momentum.
Using this it was determined that for the 1s orbital, one has the greatest probability
of finding an electron near the nucleus. That is because of the smaller radius and volume
of the atom as a whole. Thus, the farther away from the nucleus you look, the less of a
chance there is to find an electron.
To better understand electron orbitals as a whole, electron numbers have been made to
identify the electrons of an atom.
The principal quantum number (n) has values of 1,2,3… It pertains to
the period that the atom is in. Periods are represented by rows. For example, period two
has a principal quantum number of 2.
The angular momentum quantum number (l) has values of 0 to n-1.
This is related to the shape of an orbital. If l=0, the letter is s; l=1
is called p; l=2 is called d; and l=3 is known as f.
This is explained later in this reading.
The magnetic quantum number (ml) has integral values between l
and –l, including zero. For example, if the value of l is 2, then the
possible values of ml are -2, -1, 0, 1 and 2.
The electron spin quantum number (ms) can have one of two
values: +(1/2) and –(1/2). This is because only two electrons can occupy any orbital,
and they must have opposite spins.
But what do these have to do with anything? There will be more on that next.
AUFBAU PRINCIPLE
The s orbitals are represented by the Alkali and Alkali Earth Metal groups
(groups 1A and 2A, respectively). The p orbitals are the other representative
elements (3A through 8A). The d orbitals are the transition metals, excluding the
inner transition metals. Finally, the f orbitals are the inner transition metals.
According to the rules stated previously, hydrogen is in the 1s orbital. This is
because it is in the s orbital of the first period. And since it is the first atom
of the 1s orbital, it is given the distinction 1s1. Thus helium, even though it
is technically a Noble Gas, is classified as 1s2. That is because helium is the
second atom of the 1s orbital.
Lithium is in the 2s orbital. It is identified as 2s1. Since you have to add
on the previous orbitals to make it correct, the correct from is 1s22s1.
Beryllium is 1s22s2. Are you beginning to see the pattern? Boron,
which is the p orbital is also the first atom in the 2p group. Thus it is 2p1.
However, you must add the previous orbitals on to make it right, so the correct form is 1s22s22p1.
Carbon is in the form 1s22s22p2. Neon, which is at the
end of the 2p orbital, is 1s22s22p6.
The d orbital is somewhat different. Since the transition metals begin at the
fourth period, the first orbital is the 4s orbital. However, when writing the transition
metals, one must start with 3d. Why isn’t it 4d? This is because the 3d orbitals are
part of the same orbital as the 3s and 3p orbitals, but they are at a higher energy level
than 4s. That is why you have to fill the 4s orbitals before the 3d orbitals.
Therefore, the configuration of scandium is 1s22s22p63s23p64s23d1.
After filling the 3d orbitals, the 4p orbitals are filled. The two exceptions to the rule
are chromium and copper. Instead of normal configurations, they are 4s13d5
and 4s13d10 respectively. No one really knows why this happens.
Also, a shortcut to writing these configurations is substituting the highest filled
Noble Gas for its configuration. For example, the configuration of potassium is normally
1s22s22p63s23d104s1.
However one can substitute the configuration of argon with [Ar]. Thus the shorthand way to
write an electron configuration for potassium is [Ar]4s1.
For the f orbitals the rules change again. The f orbitals represent the
inner transition metals. Since it is often very difficult to explain, use the chart below
to figure out the filling order for all electrons.
Take the chart below.
--Image not done yet--
You can clearly see that the 3d orbital is higher than 4s. That is a visual
representation of the fill order. The first two electrons, representing hydrogen and
helium, respectively. It now looks like this:
--Image not done yet--
Notice that there are two arrows used. The up arrow describes the +(1/2) electron and
the down arrow describes the –(1/2) electron.
The 2s elements are lithium beryllium. Add them and you get.
--Image not done yet--
Then fill up the 2p orbitals one by one. Remember that you fill from left to right.
Therefore, there shouldn’t be any 2p orbitals with two electrons until oxygen. When
you reach neon, the whole 2p orbital is filled. That is why the Noble Gasses are so
unreactive. Their orbital subshells are completely filled, thus leaving no spots for
elements to bond with it.
This process goes on and on. Use the fill chart given above to remember what order the
electrons fill in.
HYBRIDIZATION
Hybridization is the mixing of normal atomic orbitals to form special
orbitals for bonding. It is a type of covalent bonding. For instance, the first type of
hybrid orbital is the sp3 orbital. It is named that way because it is a
combination of one 2s and three 2p orbitals. From the figure below, you can see how the
hybridization occurs.
--Image not done yet--
From the figure below for carbon we see that there are the 2s and 2p orbitals to work
with. Since carbon is sp3 hybridized, you take both the 2s and 2p
orbitals and create a new set of orbitals, sp3. This means you now have
a set of four orbitals. Since you all four electrons with you, you now have an electron in
each orbital (fill left to right). Thus, carbon can bond with up to four other elements
that can donate an electron to fill each orbital. An example of such an element is
hydrogen. The electron of hydrogen can bond with the lone electron for each of
carbon’s four hybridized orbitals. Thus, CH4 (methane) is formed.
Ethylene (C2H4) is an example of sp2
hybridization. The two carbons are double bonded to each other. For each carbon atom
three sp2 hybrid orbitals are created. Because of this, one 2p orbital
must be left behind. If you looked at this geometrically, you would see the three sp2
orbitals coming out of hydrogen with the remaining p orbital perpendicular to the sp2
orbitals. See the figure below. The sp2 orbitals, when bonded,
share a pair of electrons. As a result, two sp2 orbitals are each bonded
to a hydrogen atom and the other produces a carbon-carbon bond. These bonds are known as sigma
(s) bonds.
However, carbon is double bonded. That means that another bond needs to be made to form
the double bond. This job is left to the remaining 2p orbital. As shown in the figure
below, the p orbital creates what is known as a pi (p
) bond.
For sp hybridization, let us look again at carbon. You should already
know by now that sp means that there are two hybridized orbitals. This also means
that there are two unchanged 2p orbitals remaining. Let us take the CO2
molecule. We know that the carbon is double bonded to both oxygen atoms and that each
oxygen atom contains two lone pairs of electrons. As stated with the sp2
hybridization, sigma bonds must link up the carbon and oxygen atoms. However, since oxygen
has two lone pairs of its own, it must undergo hybridization of its own. Oxygen is sp2
hybridized. Then, since carbon has two unchanged 2p orbitals, it creates two pi bonds, one
with each oxygen atom. And since oxygen is sp2 hybridized, it will form
a p bond with the carbon atom.
By now you should realize that every double bond is made of a sigma and a pi bond.
Another example of sp hybridization is the N2 molecule. The nitrogen
molecule has a triple bond, with each atom containing a lone pair of electrons. Thus, it
must have a sigma bond and two pi bonds. The unused sp orbital contains the lone
pair of electrons.
The octet rule states that an atom must always have eight electrons in its valence
shell to be stable. However, there are exceptions to this rule. One exception is
phosphorous pentachloride (PCl5). When looking at the molecules’
structure, you notice that there are ten electrons in the valence shell. To do this, the
molecule must be dsp3 hybridized. This contains a one d
orbital, one s orbital, and three p orbitals. Each dsp3
orbital creates a sigma bond with a sp3 orbital from the chlorine atom.
The other three sp3 orbitals create the atom’s lone pairs.
The last example is d2sp3 hybridization. Sulfur
hexafluoride (SF6) is an example. By now, you should pretty much know what will
happen.
BOND ORDER & PARAMAGNETISM
Bond order is an indicator of bond strength. It is the number of bonding electrons
minus the number of antibonding electrons, all divided by two.
To find this value you set up the chart shown below. I call it a "stack
chart."
--Image not done yet--
For any molecule, for example O2, you take the total number of electrons
involved, which is 16, and place them across the chart starting at the bottom. Remember to
go left to right. Then total up the bonding and antibonding electrons and calculate the
bond order. The greater the bond order, the greater the bond strength. Also, while bond
order increases, bond energy increases and bond length decreases.
Paramagnetism is when a substance is attracted into an inducing magnetic field. Diamagnetism
is when the substance is repelled by an inducing magnetic field.
Looking at the stack chart can identify paramagnetism. If the chart shows that the
molecule has any unpaired electrons, as shown above with O2, then it is
paramagnetic.