Directing Aromatic Substituents Groups:There are
three positions on a benzene ring. This image outlines the different positiions.
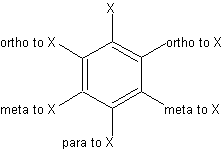
These positions are important in naming benzene ring, since its ring structure makes
numbering the carbons complicated. Let's look at a specific molecule. We have a toluene
molecule with some substituents here:
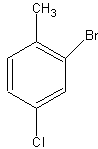
The bromine is ortho to toluene, while the chlorine is para to toluene. However, the
positions don't really matter in the naming of this specific compound. You would name this
1-bromo-3-chlorotoluene. The ortho/para/meta directing is only important when synthesizing
specific benzene-based rings. Certain substituent groups can "direct" another
substituent during certain reactions.
Directing Groups
The rate of substitution on a benzene ring is affected by what groups are already on
the benzene ring. Those groups which lower the rate of electrophilic substitution is calld
an activating group. Any substituent that lowers the rate (from benzene) of substitution
is called a deactivating group.
An important thing to know about substitution reactions on benzene is that you will
NEVER get exactly what you want. You always get a mixture of what you want (a mixture of
ortho/para/meta groups), and you must then use methods to separate the stuff you don't
want. For instance, you may want to substitute a nitro group para from the the methyl
group in the toluene. Once you have finished with the substitution reaction, only 37% of
the final product will have the nitros in the para position. About 59% will have the nitro
in the ortho position, while 4% will have the nitro in the para group.
So when you plan out your substitutions, note what is on the benzene ring and remember
that these groups will DIRECT whatever substitution you want to a certain position.
| Ortho-Para Directors |
Meta Directors |
| Strong activators |
- -NH2 (amino)
- -OH (hydroxy)
|
| Moderate activators |
- NHCOCH3 (acetamide)
- OCOCH3 (acetoxy)
- OR (alkoxy)
|
| Weak activators |
- CH3 (methyl)
- C6H5 (phenyl)
|
|
| Strong deactivators |
- -NO2 (nitro)
- -NR3 (ammonium)
- -CX3 (trihalomethyl)
|
| Moderate deactivators |
- -CN (cyano or nitrile)
- -SO3H (sulfonic acid)
- -CHO (aldehyde)
- -COR (ketone)
- -COOH (carboxyl)
|
|
Weakly Deactivating Ortho-Para
directors |
| -F |
-Cl |
-Br |
-I |
| (fluoro) |
(chloro) |
(bromo) |
(iodo) |
|
|
Why is this?
Well, electron-donating groups are activators and ortho-para directors because
they stabilize the intermediate cations. The intermediate cations are what is formed
between the removal and addition stages. Electron withdrawing groups are deactivators and met
directors because they destabilize the intermediate cations. The halogens, however, are a
weird. Halogens are "special" and so they withdraw electrons, but can also
provide electrons if they are needed.
Try some exercises:
Where would the next group be directed?



- We know that the nitro group is a strong deactivator, so it'll direct meta. The next
group would be on the meta postition.
- The -OH group is strongly activating, so the final product will be mix of ortho/para
- The -Cl group is weakly deactivating, but directs ortho/para.