SN1, SN2, E1, E2
By Jeff PeetTwo extremily common reactions in Organic Chemistry are substitution
and elimination reactions. We are going to cover four basic reactions, E1, E2, SN1, and
SN2. These reactions all compete with each other so be sure to pay attention to which
conditions fovor each reaction.
SN2
The most common is called an SN2 reaction. It is a simple nucleophilic attack of a
nucleophile onto an electrophilic carbon. This attack causes and inversion of absolute
configuration of the carbon center.
- SN2 Nucleophilic Substitution causes inversion of configuration: (best in polar aprotic
solvents, strong nucleophiles and good leaving groups.)
Going from (R) to (S)
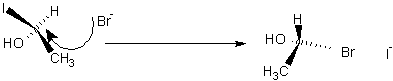
SN1
If Carbon is tertiary and nucleophile is weak then it undergoes SN1 reaction: j has same products as SN2 but is racemised and best in polar protic
solvent and the reaction is 1st order, not 2nd...
Remember:
-racemization is when there is the absolute configuration of the carbon center is not
either retained or inverted but rather randomized.
-1st order means it takes place in one rate limiting step, as opposed to the SN2 and E2
which you will learn about next, which have 2 steps.
E2
Another type of reaction is an elimination reaction. In an elimination reaction,
instead of substitute the leaving group with a nucleophile, it is removed and a double
bond is formed in the compound.
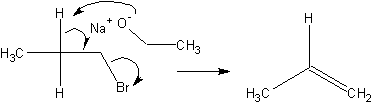
Stuff in favor of SN2 over E2: Low temperature; Modest bases, nucloephiles;
Sterically small bases; Primary is best, tertiary is worst
E1
E1 reactions occur under similiar conditions to the S1 reaction, but instead of having
substitution, elimination occurs.
E1 vs SN1: both are good with weak nucleophiles and polar protic solvents; both
want a good leaving group; tertiary substrates; if SN1, then E1 present too; these two
reactions are always competing.